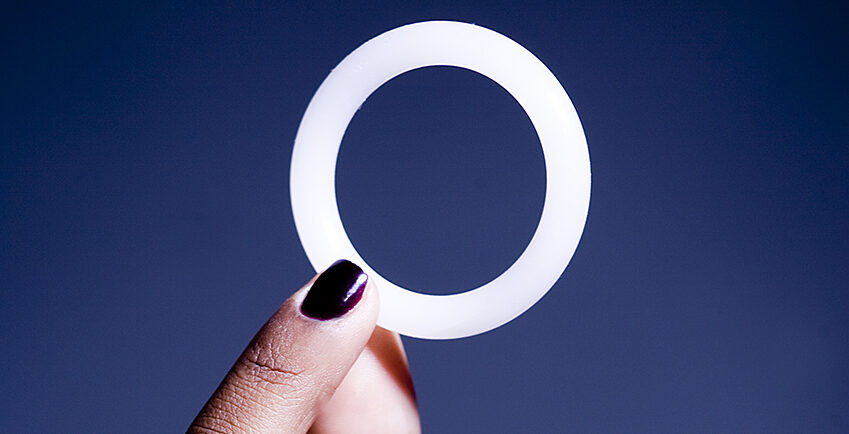
The dipivefrine vaginal ring which has been cited as a long-term HIV prevention for women has finally been approved in Kenya.
The ring which is made of flexible silicone releases anti-retroviral drug dipivefrine in the vagina over one-month period as opposed to daily pills.
Kenya joins the list of countries that have championed and cleared this method of prevention known as the vaginal ring for pilot use according to the population council measure.
The pioneers include Eswatini, Lesotho, South Africa, Uganda and Zimbabwe .
According to the Population Council measure is set to help women protect themselves from HIV infections.
A memorandum of understanding between the council and Kiara health has already been signed.
Kiara health is an African-based pharmaceutical manufacturing and health care solutions company.
Apart from the memorandum signed, the company has also received a license to locally manufacture and sell the ring which will aid to lower the cost of production and improve access.
The Council interim co- president, Jim Sailer, has said that HIV/Aids remain the biggest threat to women and therefore a need to protect themselves from it.
“Women bear the brunt of the HIV/Aids epidemic… the virus is one of the biggest threats to the health and well-being of women. In Sub-Saharan Africa, one adolescent girl or young woman becomes infected with HIV every three times… we cannot achieve the Sustainable Development Goal (SDG) of ending HIV by 2030 unless we curtail this epidemic in women. Women deserve multiple options to protect themselves against this lifelong disease” Mr. Sailer noted.
The ring can currently be used for only one month and is produced by SPS Pharma in Sweden.
The Population Council has however, noted that they are working tirelessly to introduce a three- month dipivefrine ring in order to lower annual cost and to offer women a more convenient option to protect themselves.
Once the ring is ready and submitted for regulatory approval in the next 12- 18 months Kiara Health which will receive grant funding and technical assistance for the technology transfer, regulatory certification, must oversee its access before it begins manufacturing.